
The latest events from ItaCRIN and its partners
Events
Registration is now open for our upcoming webinar!
Wednesday, December 3, 2025, Online

We are pleased to invite you to join us for a dynamic and informative session focused on WHO New Guidelines.
📅 Date: 3 december 2025
🕒 Time: 11 - 13:30
🌐 Location: Online
🔗 Registration link: https://forms.office.com/e/p2Lu4HL5rf?origin=lprLink
The primary goal of this webinar is to disseminate and analyze the new WHO guidelines on clinical trials. It offers researchers, physicians, regulators, and other key stakeholders a valuable opportunity for discussion, learning, and practical application of these standards.
In this light, the event aims to foster the implementation of the new guidelines within the European and international context, with a particular focus on best practices, ethics, and innovation in clinical research.
🎯 Whether you're involved in clinical trial design, regulation, or implementation, this webinar will provide insights and tools to support your work.
#ClinicalTrials #WHOguidelines #Webinar #ResearchEthics #Innovation #ItaCRIN #GlobalHealth #ClinicalResearch
Preliminary agenda is available
Webinar - "WHO New Guidelines for Clinical Trals: Innovation and Best Practices"
Wednesday, December 3, 2025, Online

Join us for a dedicated webinar focused on the new WHO guidelines for clinical trials.
This event will offer researchers, clinicians, regulators, and other key stakeholders a valuable opportunity to explore, discuss, and apply the updated standards.
The main goal is to promote the implementation of these guidelines across Europe and internationally, with a strong emphasis on best practices, ethics, and innovation in clinical research.
🔗 More details coming soon!
Training: 2nd Edition - Everything you need to know about submitting a European Multinational Clinical Study Proposal
Wednesday, October 1, 2025,

After the success of the first edition, ECRIN has decided to open a second edition of EYNTK about submitting an EU multinational clinical study proposal training.
Through this training, attendees will learn about the different sections of the Horizon Europe application form. The webinars will delve into different elements to consider for the submission process. To consolidate the online session, 4 parallel onsite sessions will be held in ECRIN Member countries.
This online portion of the training is open to Investigators and project managers in Horizon Europe eligible countries, considering applying for funding for clinical study projects through the Horizon Europe programme.
The eligibility requirements of the onsite trainings will include participation in the earlier webinars. The onsite sessions are planned for January/February 2026 in Madrid, Rome, Paris, and Warsaw.
The training is available at no cost to the participants. For those applying for the full training, including the webinar series and the 1 day onsite training, they will be responsible for covering the cost of their travel and accommodations to the onsite training.
All webinars are hosted on Fridays between 11-13CET.
Final dates coming very shortly.
For more information please see this page
The Role of Artificial Intelligence in Diagnostic Imaging - 2nd edition | Registration is open!
Thursday, October 16, 2025, Rome

Don't miss the 2nd edition of the event The Role of Artificial Intelligence in Diagnostic Imaging, organized by the Italian National Institute of Health (ISS), A_IATRIS, ELIXIR-IT ed EBRAINS-Italy:
📌 Oct 16, 2025
h 9:00 - 17:30
Registration to this link
For more information, please see here
REGISTRATION IS OPEN!
Friday, May 16, 2025, Online

📌 Mark your calendars and join us on May 16th 2025!
Friday, March 21, 2025, Online

The event is free of charge.
Stay tuned for the full agenda!
📣 Registration is open!
Wednesday, October 23, 2024,
The registration to the course "The Role of Artificial Intelligence in Diagnostic Imaging" is now open!
h 9:30 - 17:30
Aula Marotta, Istituto Superiore di Sanità Via del Castro Laurenziano, 10 - Rome
Registration to this link
📌 SAVE THE DATE -The Role of Artificial Intelligence in Diagnostic Imaging
Wednesday, October 23, 2024,

Mark your calendars and join us on Oct 23rd!
This event, is organised by Italian National Institute of Health (#ISS) and the Research Infrastructures Italian nodes A_IATRIS, IIB Elixir Italy and EBRAINs Italy .
The event is free of charge.
Stay tuned for the full agenda!
📣 Registration is open!
Monday, May 6, 2024,
Don't miss the next 2 events organized by the Italian National Institute of Health (ISS):
📌 May 30, 2024
Webmeeting "HORIZON EUROPE: the Research Infrastructures
for a successful proposal"
h 10 - 13:30
Registration to this link
📌 June 12, 2024
Training* "Services offered by BBMRI, EATRIS, ECRIN to support European Projects preparation - 3rd Edition"
h 9 - 17:00
Aula Marotta, Istituto Superiore di Sanità Via del Castro Laurenziano, 10 - Rome
Registration to this link
*All interested participants must attend the WEBMEETING to participate to the TRAINING.
SAVE THE DATE
Thursday, May 30, 2024,

Mark your calendars and join us on May 30th and June 12th 2024!
These two events, are organised by Italian National Institute of Health (#ISS) and the Research Infrastructures Italian nodes A_IATRIS, BBMRI.it and ItaCRIN.
The events are free of charge.
Stay tuned for the full agenda!
BIOTOOL-CHF Kick-off Meeting Marks Successful Start
Monday, January 29, 2024, Bologna

The BIOTOOL-CHF Project kicked off with a highly engaging two-day meeting on January 29 and 30, 2024
The BIOTOOL-CHF Project has been funded by the European Union in 2023 and it is coordinated by Dr. Luciano Potena. The project aims to revolutionize the management of heart failure (HF), a chronic clinical condition affecting million patients across the whole Europe. At the core of the project lies the development and validation of innovative tools and methodologies to address the challenges in HF management. From the validation of biomarkers for estimating congestion to leveraging artificial intelligence for predictive modeling, the project seeks to enhance clinical decision-making and improve patient outcomes.
Additionally, the project will develop a decision-making tool to guide the management of congestion using diuretics, along with a Point of Care companion diagnostic (CD) to assess biomarker concentrations. By integrating new tools and digital solutions, BIOTOOL-CHF aims to personalize the management of HF and optimize the use of existing pharmaceuticals. ECRIN, as beneficiary in the consortium, together with the lead CTU HCF onlus will manage a randomized controlled clinical study for the validation of the companion diagnostic strategy across ten European countries sites.
As the kick-off meeting concludes, the BIOTOOL-CHF Project looks ahead to the next phase of its journey, driven by a shared commitment to innovation and collaboration in tackling the challenges of heart failure.
For more information, please visit BIOTOOL-CHF website
SAVE THE DATE - ICTD 2024
Thursday, May 23, 2024, Greece

Mark your calendars and join us on May 23rd 2024, as we celebrate International Clinical Trials Day.
This event is organised by ECRIN (European Clinical Research Infrastructure Network), and its Greek scientific partner, GRECRIN, who will co-host this event.
This year's topic: Data Centric Clinical Research.
With the data revolution playing an increasing role in all aspects of clinical research this year we will focus on some of the impacts, benefits and challenges of the rise of data and technology. Different stakeholders from the community will share their experiences on topics ranging from the integration of the new technical assets, eHealth, data science, the data protection legislation and collaboration of academia and industry, among others.
Join the event, live or remotely, it is free!
Registration details will be made available shortly.
Training "Services offered by BBMRI, EATRIS, ECRIN to support European Projects preparation"
Monday, November 13, 2023,
📣📣📣 The registration to the training "Services offered by BBMRI, EATRIS, ECRIN to support European Projects preparation" is NOW OPEN!
IMPORTANT DATES
Course date: 6 December 2023
Deadline for applications: 24 November 2023
Registration is available here
VENUE
Aula Marotta, Istituto Superiore di Sanità, Via del Castro Laurenziano 10 - Rome, Italy
FEE
The course and registration are free of charge.
COURSE DESCRIPTION
The training proposes an overview of A_IATRIS, BBMRI.it and ItaCRIN activities as an added value in supporting and fostering biomedical research at national and European level and advices for researchers.
In the second part of the day, a practical session will be aimed to design a proposal by including the three Research Infrastructures.
TARGET AUDIENCE
The training course is open to clinicians, biologists, grant offices, principal investigators and researchers.
CONTACTS
For more information, please contact the Scientific Secretary 👉 infrastrutture_cori@iss.it
Save the Date for the next Training!
Wednesday, December 6, 2023, Rome

ISS will host the 2nd edition of the training "Services offered by BBMRI, EATRIS, ECRIN to support European Projects preparation" on 6 December 2023.
The event is open to clinicians, biologists, grant offices, principal investigators and researchers.
The training proposes an overview of A_IATRIS, BBMRI.it and ItaCRIN activities as an added value in supporting and fostering biomedical research at national and European level and advices for researchers.
In the second part of the day, a practical session will be aimed to design a proposal by including the three Research Infrastructures.
Stay tuned for the full agenda!
🗓 6 december 2023
🕒 08:30-17:00 CET
📍Istituto Superiore di Sanità, Rome, Italy
2nd EU-Africa PerMed Summer School
Wednesday, September 20, 2023,
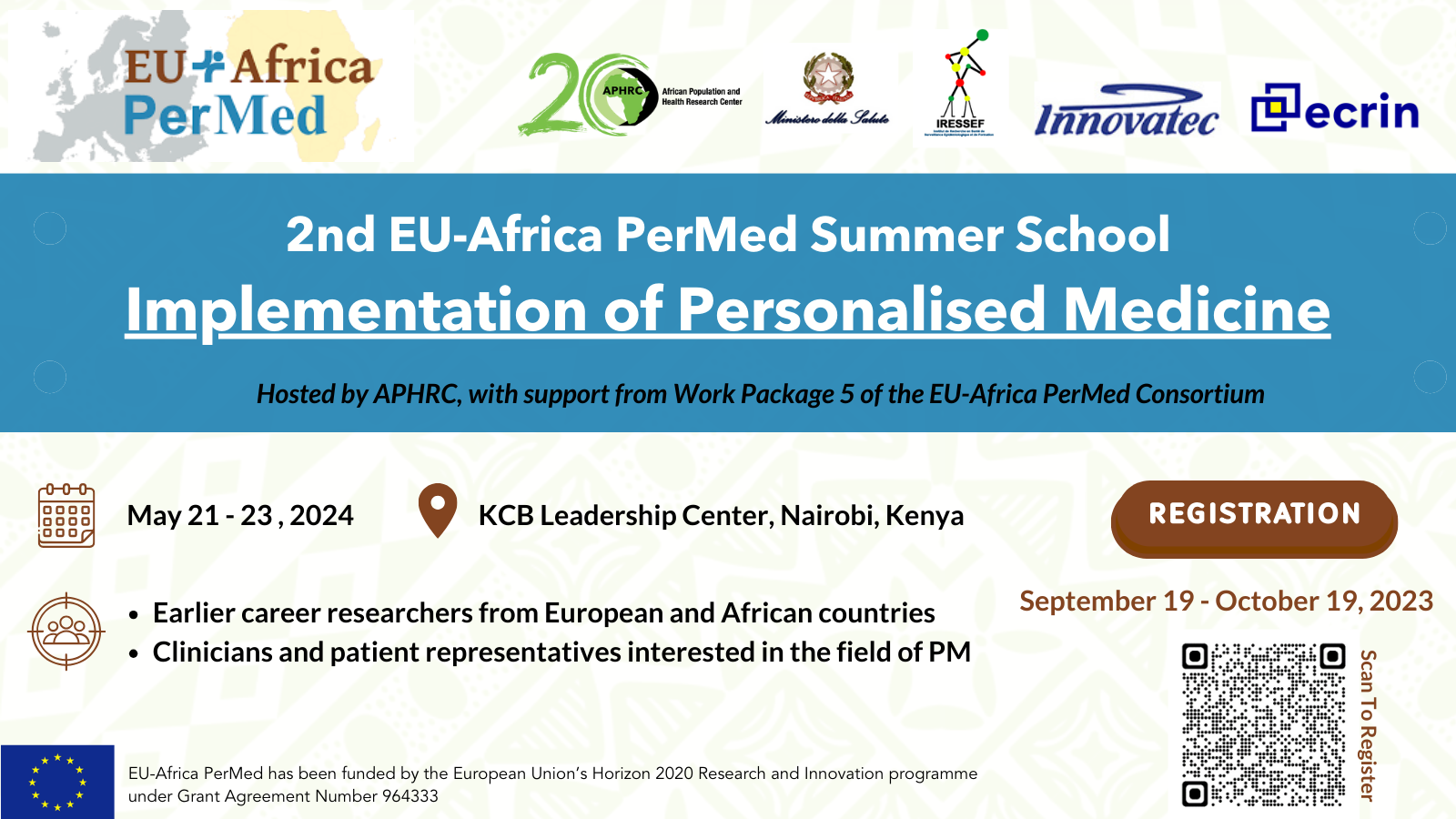
The 2nd EU-Africa PerMed Summer School: Implementation of Personalised Medicine, scheduled for May 21-23, 2024, in Nairobi, Kenya will focus on implementing PM in various fields, aiming to equip participants with skills to support PM implementation and research network creation. The Summer School (hybrid format) is open to around 35 junior researchers, policymakers, and healthcare workers from EU and African countries who will be evaluated and admitted on a competitive basis (distributed online and face to face). At least 6 participants will be selected to receive a travel and accommodation grant. Participation in this training event is free of cost.
The organization is led by the African Population and Health Research Center (APHRC) and and the Italian Ministry of Health (IT-MoH), with the collaboration of the European Clinical Research Infrastructure (ECRIN), the Institute for Health Research Epidemiological Surveillance and Training of Senegal (IRESSEF) and Innovatec.
To apply for this training event, please:
1. Read the “Applicant instructions and criteria” document below
2. Fill out the following application form here
3. Upload your CV (max 2 pages + relevant publications) in PDF format
4. Recommendation letter from your institution (mandatory only if you are applying for the grant)
🗓 21-23 May 2024
🕒 09:00 – 17:00 CEST
💻 Hybrid format
Deadline for application: October 19th
Registration is available here 👉 Registration page
☎️ Contacts
Dr Maria Jose Ruiz Alvarez: mj.ruizalvarez-esterno@sanita.it
Dr. Marta Vicente- Crespo: mvicente-crespo@aphrc.org
Rita Karoki: rkaroki@aphrc.org
For more information 👉 Call Document Draft Agenda Application Process and Evaluation Criteria
ICPerMed 2023 Training: “Research Infrastructures in Personalised Medicine: use, advantages and challenges”
Friday, September 22, 2023, Online

The aim of this training is the promotion of the use of European Research Infrastructures in the biomedical field to better inform researchers on the potential use of Biological and Medical Research Infrastructures and the benefits arising from their use, to accelerate excellence, innovation and translation, but also make them aware of the barriers to be overcome.
Although there is a focus on European Infrastructures, it might be of interest for you and your communities.
🗓 22 September 2023
🕒 09:00 – 17:30 CEST
💻 Online
Deadline for application: September 18th at 12:00 CEST
Registration is available here 👉 Registration page
For more information 👉 Click here
Training: Everything you need to know about submitting a European Multinational Clinical Study Proposal
Monday, September 4, 2023,
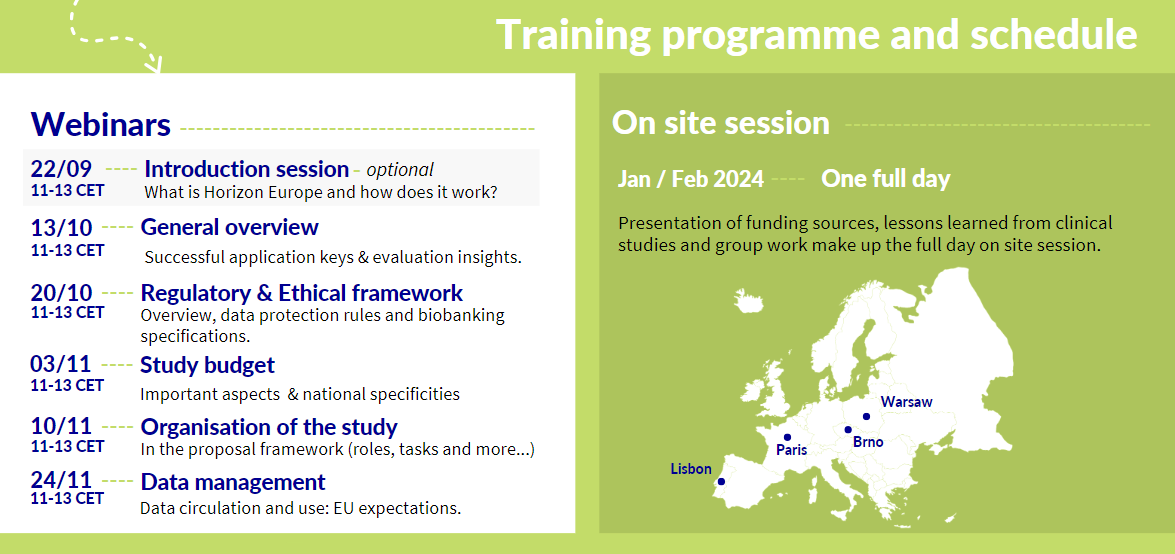
This training is open to Investigators and project managers in ECRIN Member and Observer countries considering applying for funding for clinical study projects through the Horizon Europe programme.
Individuals can either register for any of the webinars (from a single webinar through to the whole series) or they can apply to attend all webinars and the onsite training (planned pending sufficient interest in Jan/Feb 2024 in Brno, Lisbon, Paris, Warsaw). The applications for the full training can be submitted for an individual or a team (investigator & project manager).
Applications for the full training are open until September 15th, 2023. The coordination team will reach out to those accepted for the full training programme shortly thereafter.
The training is available at no cost to the participants. For those applying for the full training, including the webinar series and the 1 day onsite training, they will be responsible for covering the cost of their travel and accommodations to the onsite training.
For more information 👉 download the brochure here
EU-Permed June Webinar
Thursday, June 22, 2023, Zoom

Capacity Strengthening For Research and Implementation of Personalised Medicine
The webinar, hosted by the African Population and Health Research Center (APHRC) as part of the EU-Africa PerMed Consortium, seeks to examine PM in practice with a focus on the capacities required for the successful implementation of PM and strategies to strengthen them in the context of the African continent.
EU-Africa PerMed (Building Links between Europe and Africa in Personalised Medicine) is a four-year project funded by the European Commission H2020 Programme that has the final objective of integrating more African countries in the global PM research agenda.
🗓 22 june 2023
🕒 4:30 pm CEST
💻 Zoom
Registration is available at the link 👉 Registration page
FAIRifying sensitive data: technical, legal and practical considerations in the biomedical field
Thursday, May 25, 2023, Istituto Superiore di Sanità, Rome

We are pleased to announce the training FAIRifying sensitive data: technical, legal and practical considerations in the biomedical field organized on 25-26 May 2023 by ITACRIN at Istituto Superiore di Sanità (ISS) and it will take place in Rome.
The main purpose of this training is to introduce the principles of biological and medical sensitive data and medical applications, access to data resources and data FAIRification (especially for health research and health care data).
See the preliminary agenda here.
The registration for the training is open and it is accessible from the following link https://forms.office.com/e/sjVEy4hf7c
Services offered by BBMRI, EATRIS, ECRIN to support European Projects preparation
Friday, May 5, 2023, Istituto Superiore di Sanità, Rome

The training: "Services offered by BBMRI, EATRIS, ECRIN to support European Projects preparation" has been organized by Istituto Superiore di Sanità in collaboration with A_IATRIS, BBMRI.it and ItaCRIN.
The training proposes an overview of A_IATRIS, BBMRI.it and ItaCRIN activities as an added value in supporting and fostering biomedical research at national and European level and advices for researchers.
In the second part of the day, a practical session will be aimed to design a proposal by including the three Research Infrastructure.
🗓 5 maggio 2023
🕒 09:00-17:00 CEST
📍Istituto Superiore di Sanità, Rome, Italy
Registration is available at the link 👉 Registration page
See the programme and more information on ISS website
ICTD 2023 - SAVE THE DATE
Tuesday, May 23, 2023, Warsaw

ECRIN together with the Polish National Partner, PolCRIN (Agencja Badań Medycznych) look forward to welcoming you to ICTD 2023 in Warsaw. This year, the discussions will focus on Decentralised Clinical Trials: challenges and opportunities.
Stay tuned to the webpage for more information on the agenda and registration.
"A_IATRIS DAY: PROJECTS, COLLABORATIONS AND SERVICES"
Wednesday, November 16, 2022, Nobile Collegio Chimico Farmaceutico, Rome
The event: "A_IATRIS DAY: PROJECTS, COLLABORATIONS AND SERVICES" has been organized by Istituto Superiore di Sanità and A_IATRIS association (the Italian node of EATRIS).
The meeting proposes an overview of A_IATRIS activities as an add value in supporting and fostering biomedical research at national and European level.
In the second part of the day, a scientific session will be aimed to collaborations and interactions between young investigators by a poster session and oral presentations. Received abstracts will be published.
🗓16 November 2022
🕒 09:00-16:00 CET
📍Nobile Collegio Chimico Farmaceutico, Rome, Italy
Registration is available at the link 👉 https://forms.office.com/r/rhSbYP73XQ
To send abstracts, please click here 👉 https://forms.office.com/r/9RMUC24Uvc
See the programme and more information on A_IATRIS website
ECRIN Summer school 2022
Monday, October 3, 2022,

Last week, the first edition of the ECRIN Summer School since the outbreak of the COVID-19 pandemic took place in Villa Borghese, Rome.
ItaCRIN and all ECRIN staff came together for 3 full days of workshops and learning.
Paediatric biobanking and minor engagement
Thursday, October 13, 2022,
ESBB and BBMRI ERIC are delighted to announce a new, common community engagement activity: EUROPE BIOBANK WEEK ROADSHOW.
The EBW ROADSHOW consists of a series of smaller and regional face to face events covering a fine selection of hot topics in biobanking.
This meeting has been organized by ESBB and BBMRI ERIC in partnership with the BBMRI ERIC Stakeholder Forum Patient Pillar, the Children’s Bambino Gesù Hospital, the IDEA Network, and the National Node BBMRI it.
The meeting will kick off with an overview of European commitment to paediatric research and healthcare, followed by an in depth exchange on good practices, highlighting the priorities set by various actors to raise awareness and encourage citizen science, facilitate assent and consenting processes, and improve engagement. The first day will close with a keynote speech on the use of innovative genomic approaches with undiagnosed patients and precision medicine in children by Franco Locatelli and Marco Tartaglia. The second day features parallel breakout sessions to support the codesign of a shared recommendation on paediatric biobanking.
🗓13-14 October 2022
🕒 09:00-19:00 CEST (first day); 09:00-12:45 CEST (second day)
📍Children’s Bambino Gesù Hospital, Rome, Italy
Registration fees:
ESBB/BBMRI ERIC Member 85€
NON ESBB/BBMRI ERIC Member 160€
See the programme and register via the ESBB website today, seats are limited!
Clinical Research in the XXI century: major challenges and new opportunities
Friday, June 24, 2022,
Clinical Research is evolving and rapid changes in the last years pose numerous challenges to be overcome and many opportunities to be seized.
ClinOpsHub will host the conference as a hybrid event located in the Auditorium Hall of the Norman Swabian Castle of Mesagne (BR), in Puglia.
| 📅 24 June 2022 | |
| 🕒 09:00-17:00 CEST |
| 📍Norman Swabian Castle of Mesagne (BR) | |
| 💻or online | |
Registration: the event is free of charge however, registration is required for both on-site and online participation.
Preliminary agenda and registration form: https://www.clinopshub.com/convegno
INTERNATIONAL CLINICAL TRIALS DAY (ICTD) 2022
Tuesday, May 17, 2022,

ICTD 2022: RECRUITMENT IN CLINICAL TRIALS
Together with the KKS Netzwerk, ECRIN will host ICTD 2022 as a hybrid event located in Berlin. ICTD 2022 builds on the intentions of ICTD 2020 which was cancelled due to the pandemic and will bring together stakeholders from across Europe and the globe to discuss the challenges and opportunities related to recruitment in clinical trials. ICTD is open to research organisations, clinical research professionals, patient organisations, regulatory and ethics bodies, funders and industry.
| 📅 17 May 2022 | |
| 🕒 10:00-17:00 CEST |
| 📍Lagenbeck-Virchow-Haus, Berlin | |
| 💻or online | |
| Preliminary agenda: https://ecrin.org/events/ictd2022 |
Registration: the event is free of charge however, registration is required for both on-site and online participation. Please be careful when you arrive on the registration landing page to select the correct attendance type as they are not transferable.
BBMRI, EATRIS, ECRIN RESEARCH INFRASTRUCTURES: OPPORTUNITY AND SERVICES FOR RESEARCHERS
Tuesday, May 3, 2022,

Together with BBMRI.it, A_IATRIS, ItaCRIN, the National Institute of Health organized a Webmeeting. The main purpose of this event is to
introduce BBMRI, EATRIS and ECRIN and their activities with the aim to support and facilitate Biomedical Research in the European landscape.
The conference is addressed to biologists, medical doctors, clinical researchers, grant office and CRO/CTUs.
The official language of the Webmeeting is Italian.
The registration form is available at the page https://www.iss.it/web/guest/convegni
Please fill it in sign it and send to the Organising Secretariat by 29th April 2022.
The participation is free.
ATTENDANCE CERTIFICATE - Participants will get an attendance certificate at the end of the Meeting.
| 📅 3-5 May 2022 | |
| 🕒 14:00-16:30 CEST |
| 💻 online | |
EU-AMRI launch event
Tuesday, April 5, 2022,
EU-AMRI, the European Alliance of Medical Research Infrastructures, is the new alliance between the European medical research Infrastructures BBMRI, EATRIS and ECRIN. EU-AMRI aims to facilitate the effective and efficient use of scientific services, expertise and tools by academia and industry for the seamless translation of their scientific discoveries into new treatments and solutions for patients.
⏰ On the morning of 5 April 2022 (10:00 – 12:30 CET), EU-AMRI will be formally launched in Brussels. The official launch is a hybrid event, hosted by Vivienne Parry, and can be attended online for free.
🗓 For more information about the EU-AMRI agenda, please click https://eu-amri.org/fileadmin/user_upload/documents/EU-AMRI_Launch-event_April5-2022-def.pdf
❗️The free registration is available here https://cesnet.zoom.us/meeting/register/tJEsfuGpqzMqGtMyadjlDDJ8oyo8ew-IBwi8
II Level Master degree program - METHODS & DATA ANALYSIS (MEDAL) IN BIOMEDICAL RESEARCH
Friday, January 28, 2022,

The Department of Medicine & Surgery of University of Milano - Bicocca is pleased to announce the launch of a new II Level Master degree program - METHODS & DATA ANALYSIS (MEDAL) IN BIOMEDICAL RESEARCH.
It is a unique program designed to train students to work in modern medical statistics and data science environment with expertise in study design, statistical and computational approaches and advanced data analysis methodologies in the evolving field of Biomedical Research.
The program offers a structured pathway in 12 months, with a total of 60 ECTS (35 for courses, 20 for stage, 5 for thesis). The program involves a strong partnership with Industry, with some of the leading companies participating as Associate Partners in the program, and with Research Centers. The program courses will mainly be offered in remote learning and internships will be customized, based on the interest of the students. Deadline for application: February 25th, 2022
Start of the Master program: April 4th, 2022
Tuition fee: 4000 Euro
In order to get a better insight into the program: https://mastermedal.unimib.it/
Book an Info Session with the Course Director & Vice-Director: mastermedal@unimib.it
Save the date: ECRIN CTU Day 26 November 2021
Friday, October 1, 2021,
ECRIN aims to strengthen collaboration and open the dialogue among the CTUs from the CTU networks in ECRIN’s Observer/Member countries. For this reason, we are pleased to announce the first ECRIN CTU day will be held from 10:00-13:00 CET 26 November 2021 online. It is open to personnel from CTUs of ECRIN’s national partners. Details on ECRIN’s activities and its regular collaboration with CTUs and national hubs will be highlighted. A practical use case will cover a variety of aspects related to the setup and management of a multinational clinical trial. Stay tuned for more details and visit ECRIN CTU Day page.
PedCRIN final event
Friday, June 4, 2021,
The PedCRIN (Paediatric Clinical Research Infrastructure Network) consortium will host a final event “Fostering International Paediatric Clinical Research” on June 16th 2021, from 9:30 to 13:00 CEST. The free online event will bring together stakeholders in paediatric and neonatal clinical research to discuss the key outcomes and accomplishments. PedCRIN comes to an end on June 30th 2021 and there is much to share from this project which includes direct support to clinical trials and the development of tools to facilitate clinical research in the paediatric and neonatal communities.
Contact information
For more information on PedCRIN or the Final Event: Fostering International Paediatric Clinical Research, visit the PedCRIN website or contact PedCRIN via mail at pedcrin@ecrin.org
PedCRIN website: http://www.pedcrin.org
Registrations for the final event: Link
Don't miss International Clinical Trials Day: 20 May 2021
Wednesday, April 14, 2021,

In light of the current pandemic, ECRIN is organising a virtual event for International Clinical Trials Day (ICTD) on May 20th, 2021.
ICTD 2021 will bring together stakeholders from across Europe and the globe to discuss the growing interest in platform trials from best practices to challenges in the European context. ICTD is open to research organisations, clinical research professionals, patient organisations, regulatory and ethics bodies, funders and industry.
The theme of this year's event is Platform Trials: Shift in Treatment, Testing and Collaboration. This new trial format raises many questions, changes our approach to conducting clinical trials and testing for appropriate treatments.
The virtual event (10:00 – 16:00 CEST) is free and open to all.
REGISTRATION IS NOW OPEN on the ECRIN website
JOIN US! May 20th 2021 is International Clinical Trials Day
Thursday, February 18, 2021,

On this day we commemorate the launch of James Lind's clinical trial which laid the groundwork for modern-day clinical research. This year we will bring together stakeholders from across Europe and the globe to discuss the growing interest in platform trials from best practices to challenges in the European context.
Registration opening soon 👉 https://ecrin.org/events/ictd2021
EMA two-part CTIS training event targeted at SME and academia
Wednesday, January 20, 2021,
A two-part CTIS training event targeted at SME and academia clinical trial sponsors (future CTIS users and their organisations).
Day 1: 22 Feb 2021 SME and academia Clinical Trials Information System (CTIS) two-part training webinar: Day 1 | European Medicines Agency (europa.eu)
Day 2: 4 Mar 2021 SME and academia Clinical Trials Information System (CTIS) two-part training webinar: Day 2 | European Medicines Agency (europa.eu)
The way clinical trials are conducted in the EU will undergo a major change when the Clinical Trial Regulation (Regulation (EU) No 536/2014) comes into application. The Regulation harmonises the assessment and supervision processes for clinical trials throughout the EU, via a Clinical Trials Information System (CTIS). CTIS will contain the centralised EU portal and database for clinical trials foreseen by the Regulation and will be used by clinical trial sponsors as a single entry point in the EU to obtain approval for clinical trials based on applications and for monitoring clinical trials during their life cycle, including the submission of summary of results.
CTIS will centralise the submission process for clinical trial applications and the assessment and authorisation by Member States in a single unique platform. It will facilitate day-to-day business processes of Member States and sponsors of clinical trials throughout the lifecycle of a clinical trial harmonising submission and maintenance of trial applications, assessment and supervision of trials and promoting patient safety and transparency.
The Clinical Trials Regulation, Regulation (EU) No 536/2014, will become applicable as CTIS goes live, which is anticipated in December 2021. Once launched, CTIS will be immediately available for authorities and for clinical trial sponsors, while a three-year phased transition period from the current Directive 2001/20/EC to the Regulation will apply.
To facilitate preparedness for use of CTIS users, the European Medicines Agency has developed a training programme to provide future CTIS users with the skills and knowledge to successfully use CTIS into their business. A wide array of comprehensive online training material will be made available gradually in 2021.
For Micro, Small to Medium Enterprises (SMEs) and academia, EMA is organising a targeted webinar to provide training on CTIS and its functionalities. The webinar is divided into two parts (Day 1 and Day 2) and participants are advised to attend both. This two-part event is free of charge.
As per current planning, Day 1 on 22nd February 2021 will present CTIS access management, user management and different roles in CTIS. Day 2 on 4th March 2021 will focus on CTIS functionalities - how to submit and manage a clinical trial application. Transparency of reporting of results will also be presented on Day 2.
The webinar will include presentations by speakers from EMA system experts and presenters from SME and academic institutions, demonstration of the system, and opportunities for questions and answers. The webinar will be recorded and published for future perusal.
❗️❗️Steps to take:
1) Express your initial interest in this two-part CTIS training event aimed at SMEs and academia by completing the expression of interest questions: EUSurvey - Survey (europa.eu). This initial expression of interest should be made by the 29th January 2021 but is not yet the registration to the training.
2) Based on expressions of interest, you will receive a separate email from EMA including a link to register for the training in the week commencing on 1st February 2021. Registrations will close on 7th February 2021. You will need to register for both dates individually.
Due to the platform used, this two-part CTIS training event is limited to 950 participants. Should more participants express interest to participate, preference will be given to those representing academic institutions and SMEs. The criteria for being classified as an SME can be found here.
- EMA support for SMEs, please see https://www.ema.europa.eu/en/human-regulatory/overview/supporting-smes
- EMA engagement with academia, please see https://www.ema.europa.eu/en/partners-networks/academia
Research Data Management and Open Data Course
Thursday, June 4, 2020, Webinar
APRE training programmes provide potential applicants/eligible participants in the European Commission programmes with support throughout the entire project life cycle: from project idea to management, reporting and exploitation of project results.
The training course on "Research Data Management and Open Data" is divided into 4 webinars.
The aim of the course is to provide tools for the correct management of research data and open science cloud (EOSC).
For more information, please visit APRE website.
Workhsop - Roadmap Initiative to Good Lay Summary Practices
Wednesday, January 15, 2020, Crowne Plaza Le Palace, Brussels, Belgium
Learn about the draft Lay Summary Best-Practice Guideline elaborated in response to the Clinical Trial Regulation and take the opportunity to impact the version that will be disseminated for public consultation
The upcoming Clinical Trial Regulation requires the development and dissemination of Lay Summaries of clinical study results from commercial and non-commercial sponsors. Several large companies have already generated experience with creation of Lay Summaries and in the USA guidance on Lay Summary content have already been developed.
Also, EMA has released an Expert Group Recommendation on the content of Lay Summaries. However, there is no guidance for all types of sponsors on how best to handle the development process of Lay Summaries and how best to ensure reliable dissemination so that the ultimate goals can be achieved: increase of clinical research transparency, patients’ and public’s understanding of clinical research, as well as feedback to study participants about the results of their study.
To create a suitable, mutually acceptable framework for Lay Summaries a consorted effort of all involved stakeholders is required.
Some of our keynote speakers and faculty will be:
Edit Szepessy, DG SANTE, EU Commission
Till Bruckner, TranspariMed
Kaisa Immonen, EPF
Barbara Bierer, MRCT Center, Harvard Medical School
Debra Gueirrero, Janssen
Nicola Ruperto, Ospedale Pediatrico Istituto Gaslini
Sabine Kläger, ECRIN
Thomas Schindler, Boehringer Ingelheim
Begonya Nafria Escalera, San Juan de Deu Hospital
Lotte Klim, EUPATI Fellow
Amanda Hunn, AJ Associates
Behtash Bahador, CISCRP
Sini Eskola, EFPIA.
Still time to register! Click here: https://form.jotformeu.com/93291775221358
Facilitating High Quality Multinational Clinical Research in Europe: ECRIN Mission and Vision
Wednesday, October 23, 2019, Istituto Superiore di Sanità

A meeting entitled 'Facilitating high-quality multinational clinical research in Europe: ECRIN mission and vision' will be held at the Istituto Superiore di Sanità (ISS) on 23 October 2019.
ISS coordinates the Italian Clinical Research Infrastructure Network (ItaCRIN), which aims to sustain independent clinical research and help investigators/sponsors promote (and join) multinational trials supported by ECRIN.
The purpose of the event is to improve knowledge of ECRIN and to encourage Italian participation in international trials, by promoting high-level, non-profit clinical research focused on the identification of innovati ve therapetuic strategies for the the benefit of public health.
The conference is aimed at researchers and clinical investigators, medical doctors, grant office staff, clinical research organisations (CROs), and clinical trial unit (CTU) staff.
Participation is free-of-charge, but registration is required.
Learn more on the ISS website, or contact Maria Buoncervello.
Giornata di lancio bandi H2020 – Infrastrutture di ricerca: last call 2020
Tuesday, October 22, 2019, MIUR, Viale Trastevere 76/A Sala Aldo Moro (Ex Sala della Comunicazione), Rome

Organized by APRE, MIUR
Istituto Superiore di Sanità
Viale Regina Elena, 299 - 00161 - Roma
